Improving lithium-ion battery capacity

The search for a better lithium-ion battery—one that could keep a cell phone working for days, increase the range of electric cars and maximize energy storage on a grid—is an ongoing quest, but a recent study done by Canadian Light Source scientists with the National Research Council of Canada showed that the answer can be found in chemistry.
“People have tried everything at an engineering level to improve batteries,” said Dr. Yaser Abu-Lebdeh, a senior research officer at the NRC, “but to improve their capacity, you have to play with the chemistry of the materials.”
Lithium batteries have electrodes composed of graphite and a binder called polyvinylidene difluoride (PVDF), explained Abu-Lebdeh, who worked with NRC colleagues and scientists at the CLS on the study. Silicon, however, has 10 times more lithium capacity than graphite so there has been a focus on creating a composite electrode made of both silicon and graphite.
This appears to be a viable path to increasing the energy density of rechargeable lithium ion batteries, but the silicon composite batteries lose capacity very quickly during cycling; after just five cycles, their capacity was even lower than graphite-only electrodes, even for small amounts of silicon in the composites.
“The project would have been very challenging to complete without the CLS’s ability to see the chemistry of the interface at the nanoscale,” says CLS industrial scientist Jigang Zhou, a battery expert on the research team.
The team used several NRC and CLS facilities, including electrochemical testing, scanning electron microscopy and X-ray spectroscopy, to develop a new battery characterizing method that demonstrates the chemistry at the surface of the battery. The technique could be used for research in a variety of rechargeable batteries, which Zhou is excited about.
“For me, the true beauty is that this new characterizing method is a beam to new understanding of so many battery research questions,” says Zhou.
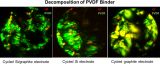
The team applied their technique to understand why even a small amount of silicon, when combined with graphite and the binder, caused the batteries to degrade. The problem, they discovered, was the decomposition of the PVDF binder during battery cycling, which occurred with graphite-only electrodes despite good battery performance.
Their results, published by the American Chemical Society online in an open access paper, provide a guide to the design of more suitable binders for composite graphite/silicon electrodes, new polymers that display no adverse chemical interaction with either component, pointing to the possibility of increasing battery capacity by including silicon.
Abu-Lebdeh expects to see the development of new binders leading to an incremental increase in silicon content, to a target of about 20 per cent. The result, he said, would be higher energy density batteries with three times more capacity than those with graphite-only electrodes. He added that while adding silicon increases battery capacity, it does not increase the cost if it is integrated as a non-disruptive, drop-in technology into current manufacturing processes.
Abu-Lebdeh credits Zhou and CLS scientist Jian Wang with providing “the fundamental understanding that was critical to this work. It was truly collaborative research, and they provided a crucial piece to the puzzle.”
Abu-Lebdeh described the researchers’ findings as an important consideration for the lithium-ion battery industry that is working toward cost-effective solutions for next-generation consumer electronics, electric vehicles and power grids.
Story by Colleen MacPherson
Source of information
Canadian Light Source & Profibusiness.world
Date
January 07, 2019